Draw the Lewis Structure for the Pcl+4 Ion
Lets consider the Lewis structure for CCl 4. The understanding of chemical reactions and physical properties can be better understood with the visualization in a 3-dimensional platform.
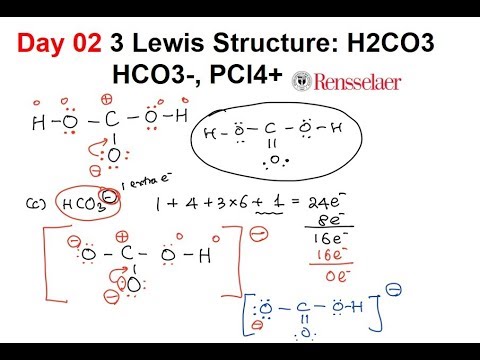
Day02 3 Lewis Structure H2co3 Hco3 Pcl4 Youtube
Drawing the Lewis Structure for PCl 4-Viewing Notes.

. Find the required count of electrons needed to make the atoms complete. Lewis structure of NO. N3-What is formal charge.
The formal charge on each atom is. Draw the Lewis structure for each molecule or ion. A simple estimate of how the arrangement of electrons around an atom changes when it forms chemical bonds.
Following VSEPR rule steps are followed to draw the lewis structure of NO and they are explained in detail in. SO3 and PCl5 despite being hypervalent molecule acts as electrophile due to the high electronegativity of surrounding atoms who tends to pull the bonded pair of electrons more precisely electron cloud towards themselves thus leaving the sulphur or phosphorus atom electron deficient. Draw the Lewis structure for each compound.
NOCl CF 2 Cl 2 HCN. We can write two possible structures. When drawing the lewis structure for ICl4 we count up the valence electrons 747136 and add the octets of electrons to the outer atoms 8 electrons to each of the 4 Cl32 used electrons which gives us a remaining 4 electrons 3632.
To compare the number of valence electrons near an atom when its bonded to when its not. There are a total of 28 valence electrons for the BrF 3 Lewis structure. Calculate the total number of valence electrons present.
Find more Chemistry widgets in WolframAlpha. Commonly Tested Lewis Structures C 2 H 2 C 2 H 4 CH 4 ClO 4- CS 2 HCl H 2 O N 2 NH 3 NO 3- O 2 OF 2 SO 3. Determine the electron pair geometry of the molecule.
Draw the Lewis structure for a molecule of each of these compounds and io Add To Playlist. 05012017 Chemical Bonding Naming the shape of molecules with one. C l C l P C l C l Student Name.
The hybridization of I3 Triiodide ion is sp3d. The bond angle for tetrahedral structures is 1095 degrees. Draw the Lewis structure for each ion.
If you add up all of the electrons in hypothetical PCl 4 15 17 17 17 17 83-. Most stable lewis structure of NO is shown below. Find the total valence electrons for the molecule.
O 6 4 - ½4 0 Bottom structure. Could someone please explain to me how to do both of these things. Get the free Lewis structure widget for your website blog Wordpress Blogger or iGoogle.
What is the lewis structure for PCL4-. Previous question Next question. Im somewhat confused because the Lewis Structure makes it seem like the shape will be linear and the angle will be 180 degrees but according to the solutions manual the shape is angular.
View Homework Help - ALEKS9html from CHEM 1320 at University of Missouri. XeO 2 F 2. Read my article in Science Education based on my dissertation.
Find the total count of valence electrons to molecules. H 2 S NCl 3 OH -. Also there is an unpaired electron on nitrogen atom.
So hypothetical PCl 4 has a charge 83 - 83 0. The most convenient way is shown here. Following steps are required to draw ClO 3-lewis structure and they are.
If you add up all of the protons in hypothetical PCl 4 15 17 17 17 17 83. In this step add the total count of valence electrons from all the atoms in a bit. Determine the central atom in this molecule.
Bonding electrons which are shared by a pair of atoms and nonbonding electrons which belong to a particular atom but do not participate. We draw Lewis Structures to predict. There is a double bond between nitrogen and oxygen atom.
Put the least electronegative atom in the center. A HC2- ion In HC2- ion both C bears sp hybridization. View the full answer.
Steps of VSEPR rule to draw lewis structure of NO. Chemistry questions and answers. Steps for Writing Lewis Structures.
For problem 419c it asks you to predict the shape and estimate the bond angle of BH2-. After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets. You will learn all steps and rules of lewis structure drawing.
The way we draw the structure of molecular compounds on paper is just a two-dimensional form. In the Lewis structure for PCl 4-there are a total of 34 valence electrons. What is the general idea of a formal charge.
After then define the number of bonds in the given molecule. Thus the Lewis structure of NO is. List the four orbitals of each C and what they are doing sigma bond pi bond lone pair empty HC2 CH2CH3 Sp ơ bond to Hs.
Now we can measure the bond angle between two of the phosphorus - chlorine bonds. Draw the lewis structure for Azide. Steps of drawing ClO 4-lewis structure.
Each chlorine at no. N 5 - 3 - ½4 0. In the PCl 4-Lewis structure Phosphorus P is the least electronegative so it goes in the center.
O 6 - 3 ½4 1 The best Lewis structure is one that has the fewest formal charges the top structure. Drawing the Lewis Structure for BrF 3. To generate the Lewis dot structure you have to follow the given steps.
Four pairs will be used in the chemical bonds between the P and F. Now we are going to learn how to draw the lewis structure of ClO 4-ion step by step. We can draw the Lewis structure on a sheet of paper.
CH 4 NH 3 I 2. If we draw a Lewis structure of a Phosphorus tetrachloryl ion PCl4 the geometry of the ion is tetrahedral. N 5 4 - ½4 -1.
Thus lewis structure is done. Draw the Lewis structure for the molecule. For this we need to do the following steps.
H always goes outside. Notice that there are two kinds of electron groups in this structure. For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule.
Draw the Lewis structure for BH _3 0714. PCl 4-is a negative ion an anion so you have to add an extra valence electron. Put two electrons between atoms to form a chemical bond.
To do so we first need to draw a Lewis structure for PCl 4. Drawing the Lewis Structure for BrF 3. Lewis Structure for I 3-A Step-by-Step Tutorial.
17 has 17 protons and 17 electrons so overall no change. Step-by-step tutorial for drawing the Lewis Structure for I3-. Draw the Lewis structure for each molecule.

Shapes Of Molecules And Ions Ppt Download

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Drawing The Lewis Structure For Pcl4 Youtube

How To Draw The Lewis Structure For Pcl4 Youtube

The Shapes Of Pcl 4 Pcl 4 And Ascl5 Are Respectively

What Is The Total Number Of Valence Electrons Represented In The Lewis Structures Of Each Of The Following Molecules Or Molecular Ions Draw Their Lewis Diagrams Before Responding A Hocl Molecule B

Consider The Phosphorus Tetrachloryl Pcl4 Cation What Is The Central Atom Enter Its Chemical Symbol Study Com

Pcl4 Lewis Structure How To Draw The Dot Structure For Pcl4

Pcl4 Phosphorus Tetrachloryl Ion Molecular Geometry Bond Angles And Electron Geometry Youtube





Comments
Post a Comment